Caesium »
PDB 7jqw-8rsy »
7lkl »
Caesium in PDB 7lkl: The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
Enzymatic activity of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
All present enzymatic activity of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring:
4.2.1.20;
4.2.1.20;
Protein crystallography data
The structure of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring, PDB code: 7lkl
was solved by
E.Hilario,
M.F.Dunn,
L.J.Mueller,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.92 / 1.05 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 181.741, 59.81, 67.299, 90, 94.65, 90 |
| R / Rfree (%) | 15.5 / 17.6 |
Other elements in 7lkl:
The structure of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring also contains other interesting chemical elements:
| Fluorine | (F) | 3 atoms |
| Chlorine | (Cl) | 4 atoms |
Caesium Binding Sites:
The binding sites of Caesium atom in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
(pdb code 7lkl). This binding sites where shown within
5.0 Angstroms radius around Caesium atom.
In total 8 binding sites of Caesium where determined in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring, PDB code: 7lkl:
Jump to Caesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Caesium where determined in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring, PDB code: 7lkl:
Jump to Caesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Caesium binding site 1 out of 8 in 7lkl
Go back to
Caesium binding site 1 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
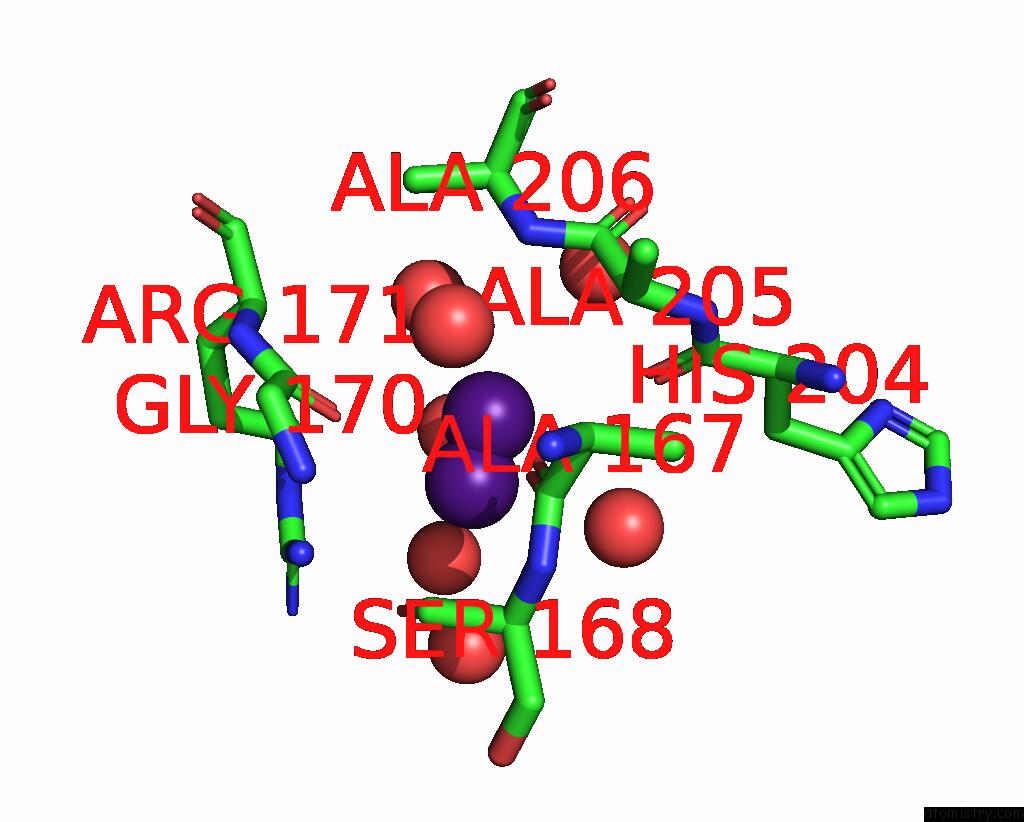
Mono view
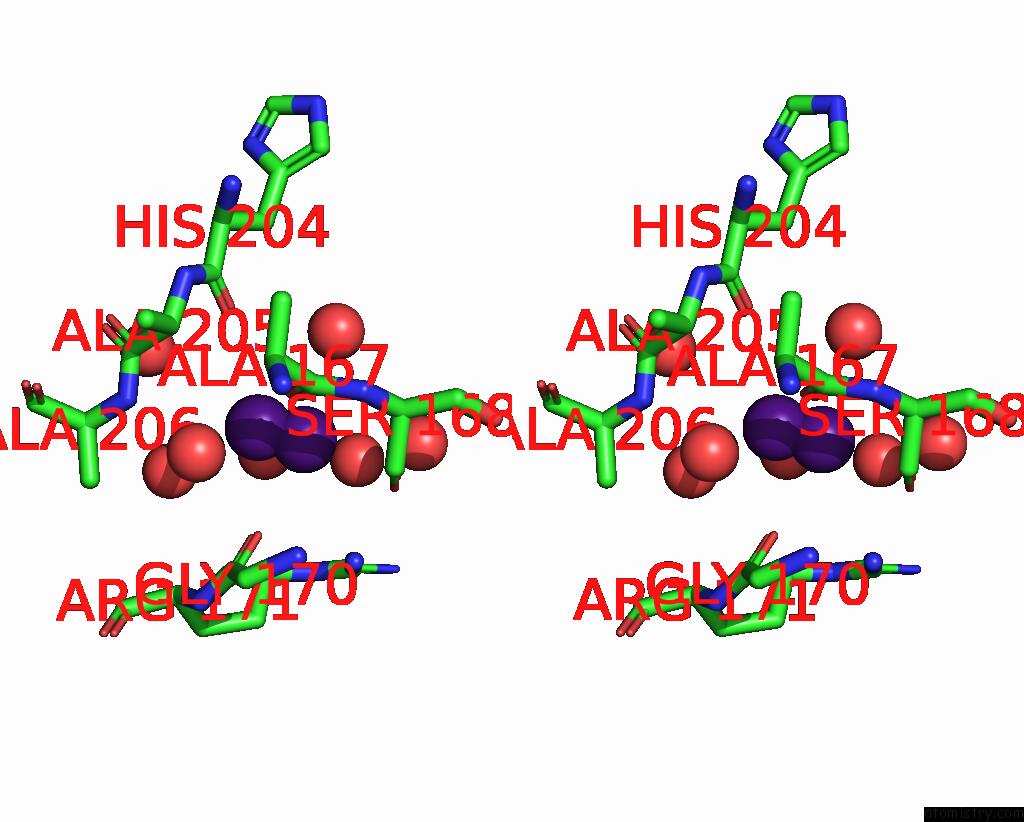
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 1 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 2 out of 8 in 7lkl
Go back to
Caesium binding site 2 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
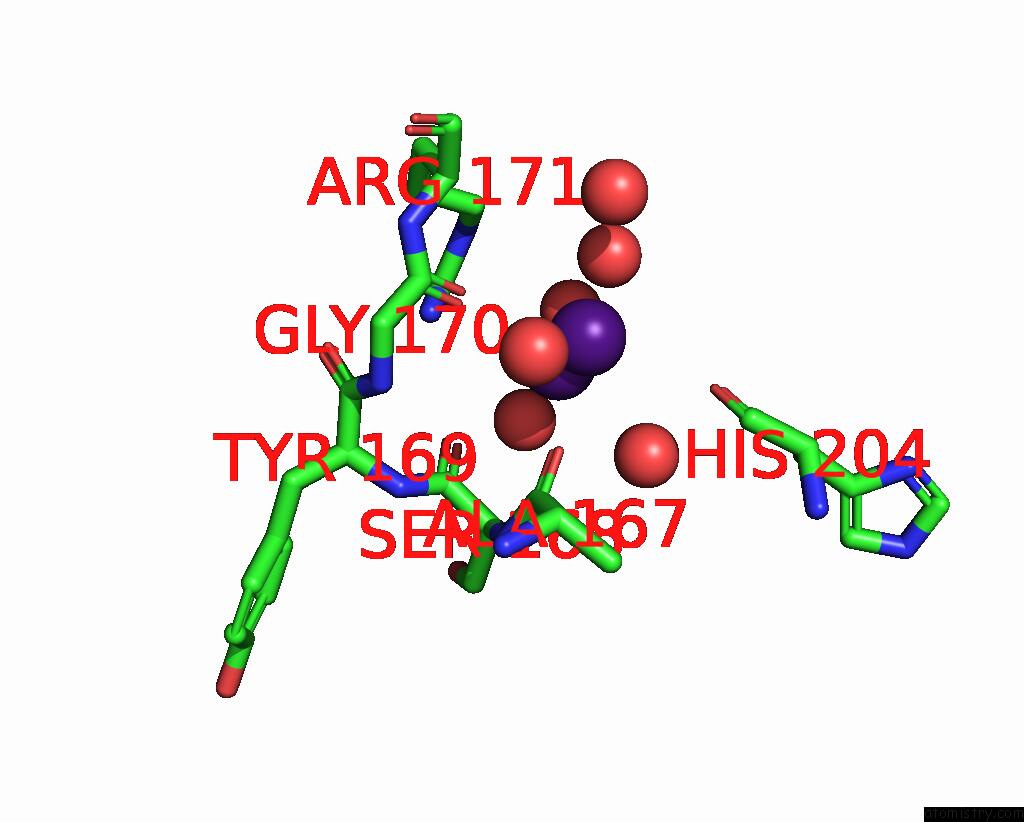
Mono view
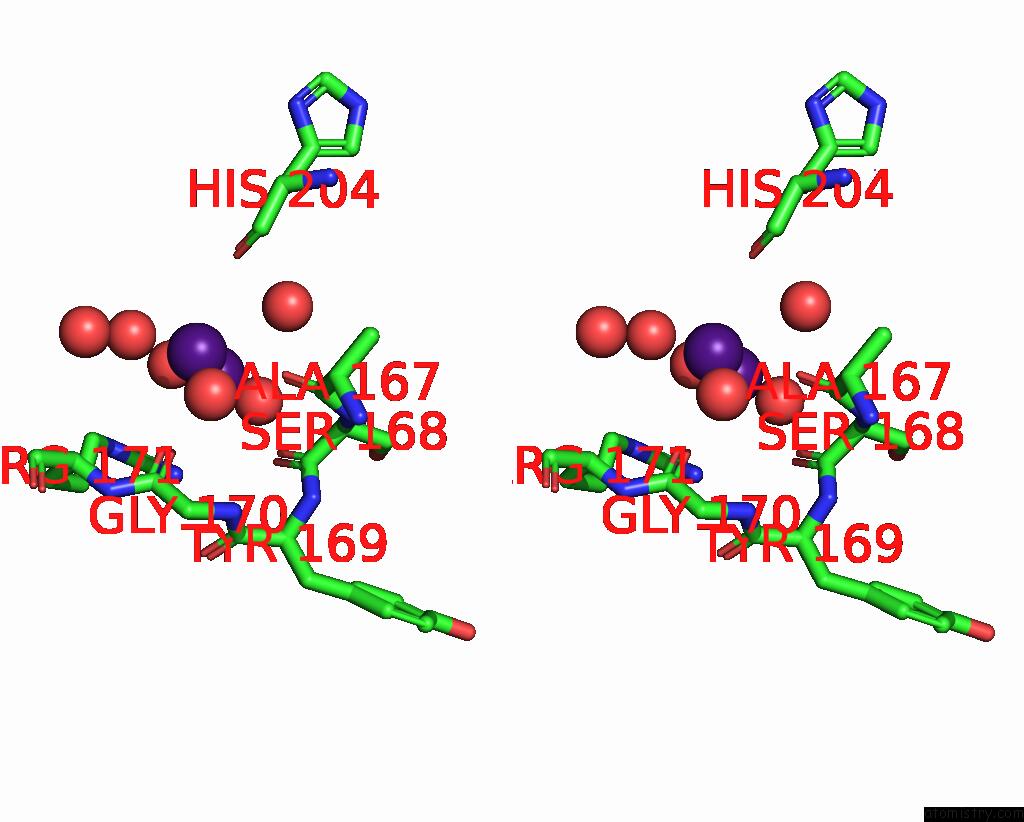
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 2 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 3 out of 8 in 7lkl
Go back to
Caesium binding site 3 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
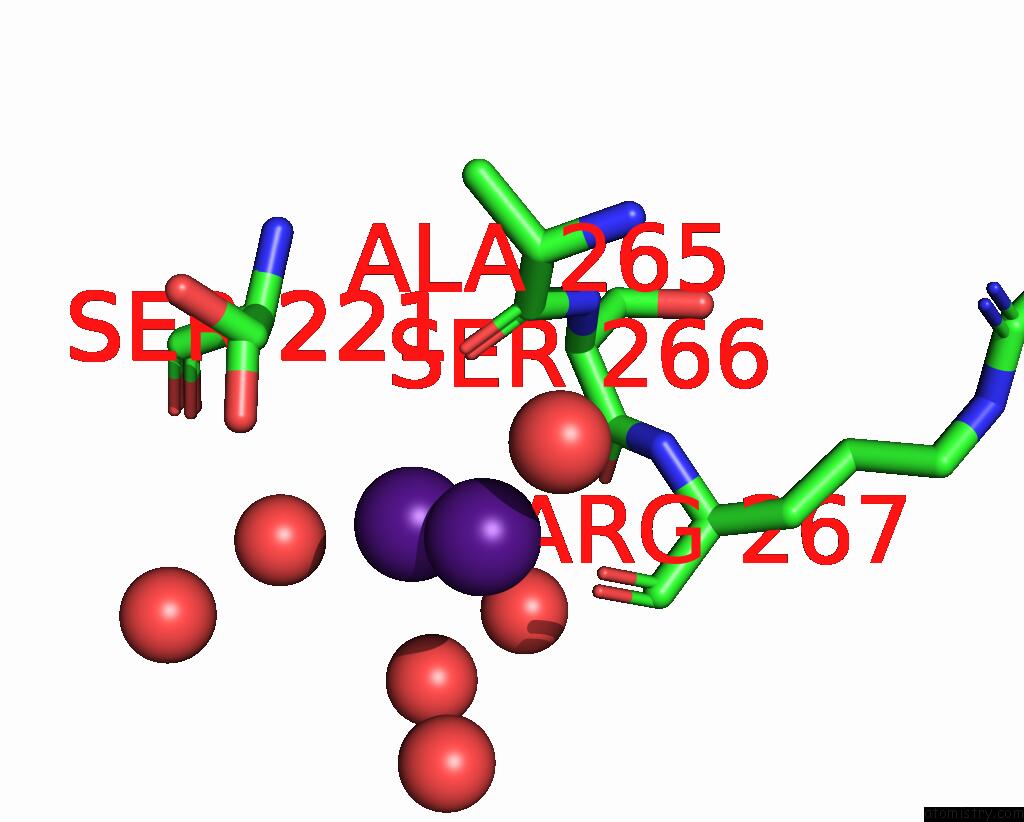
Mono view
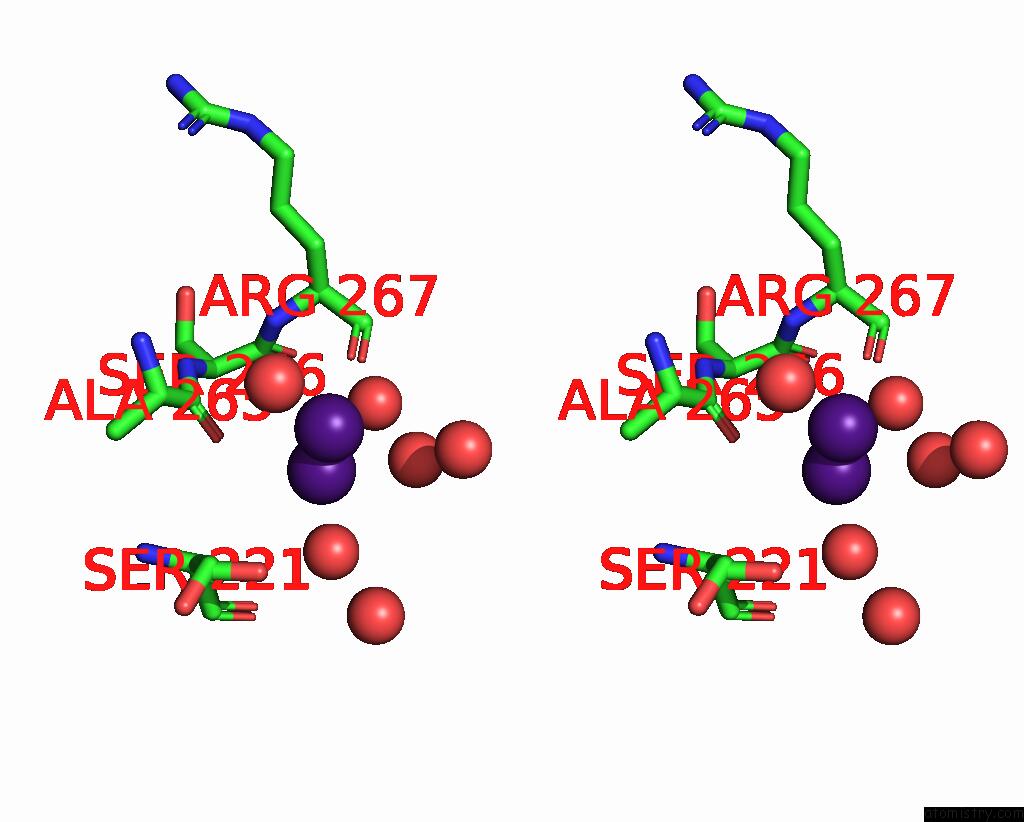
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 3 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 4 out of 8 in 7lkl
Go back to
Caesium binding site 4 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
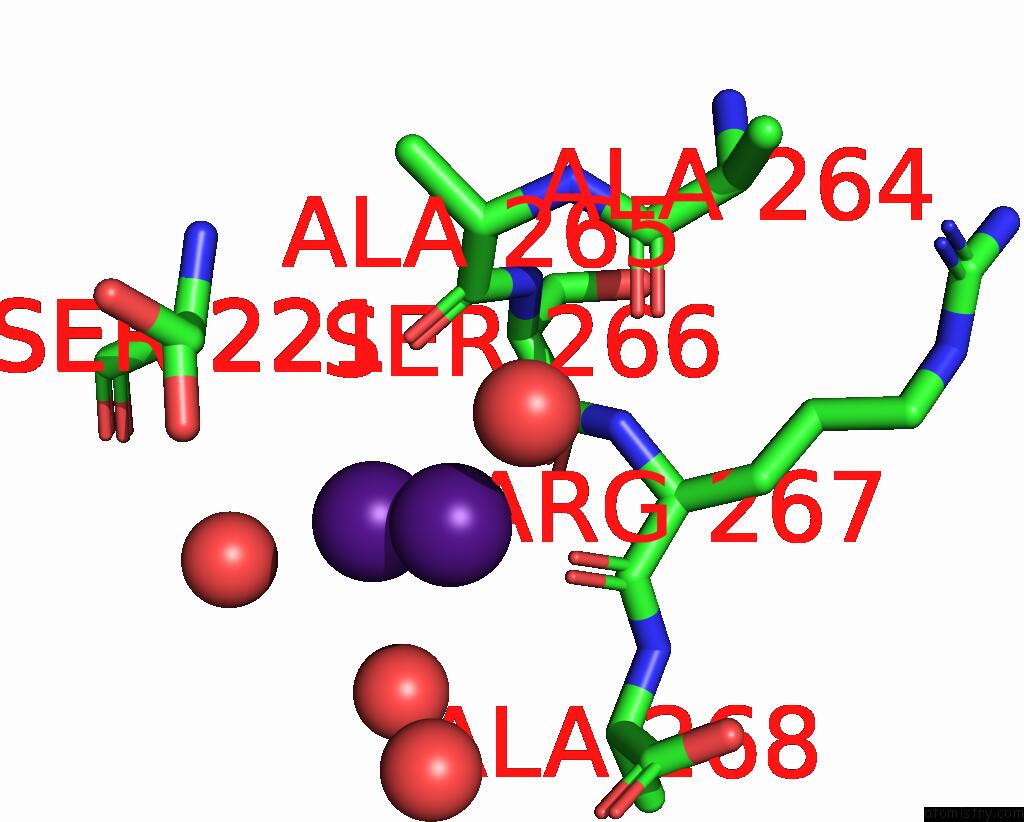
Mono view
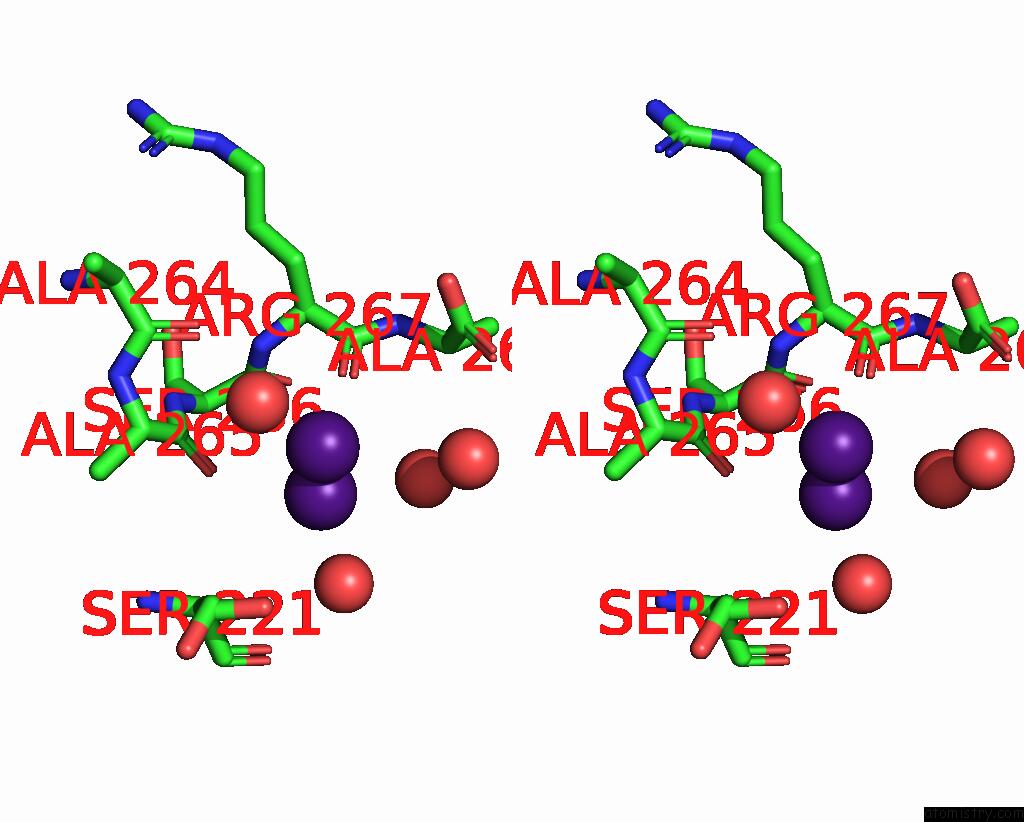
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 4 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 5 out of 8 in 7lkl
Go back to
Caesium binding site 5 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
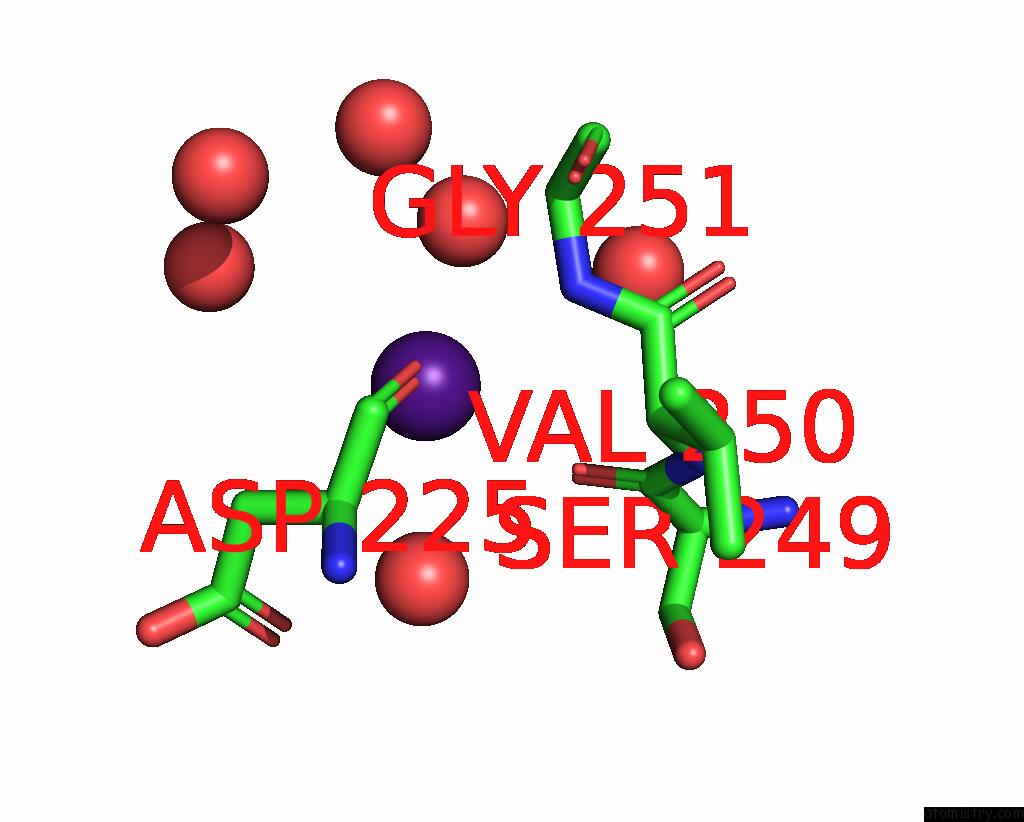
Mono view
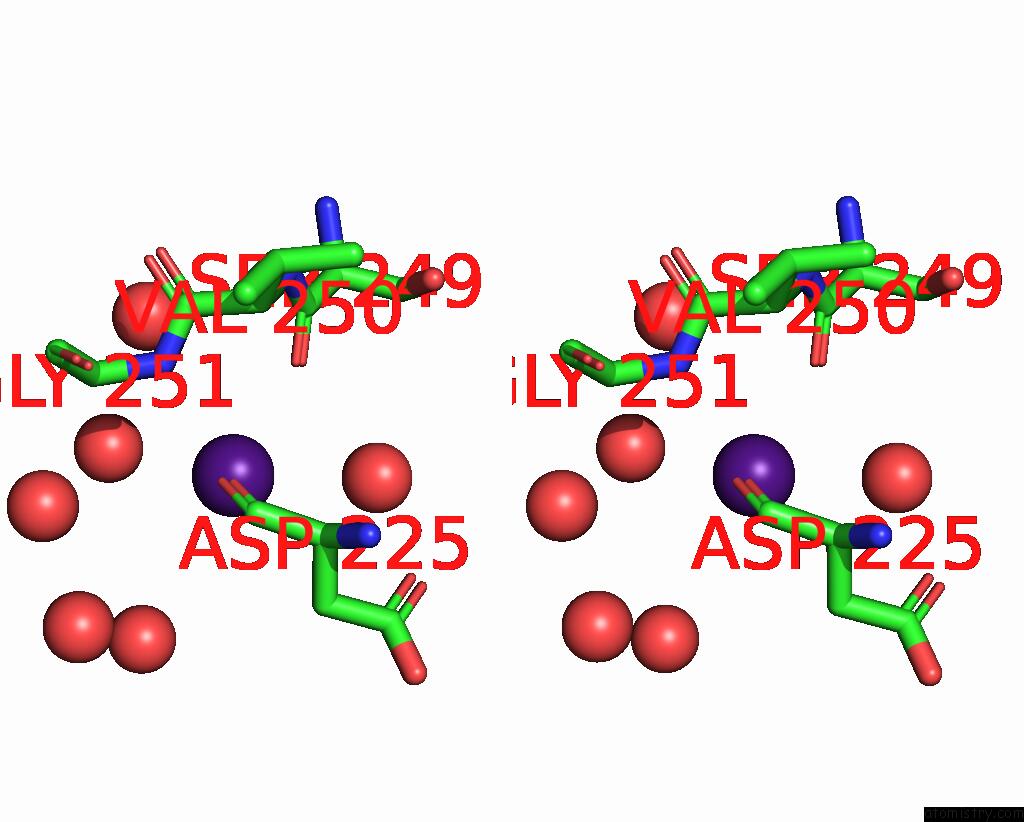
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 5 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 6 out of 8 in 7lkl
Go back to
Caesium binding site 6 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
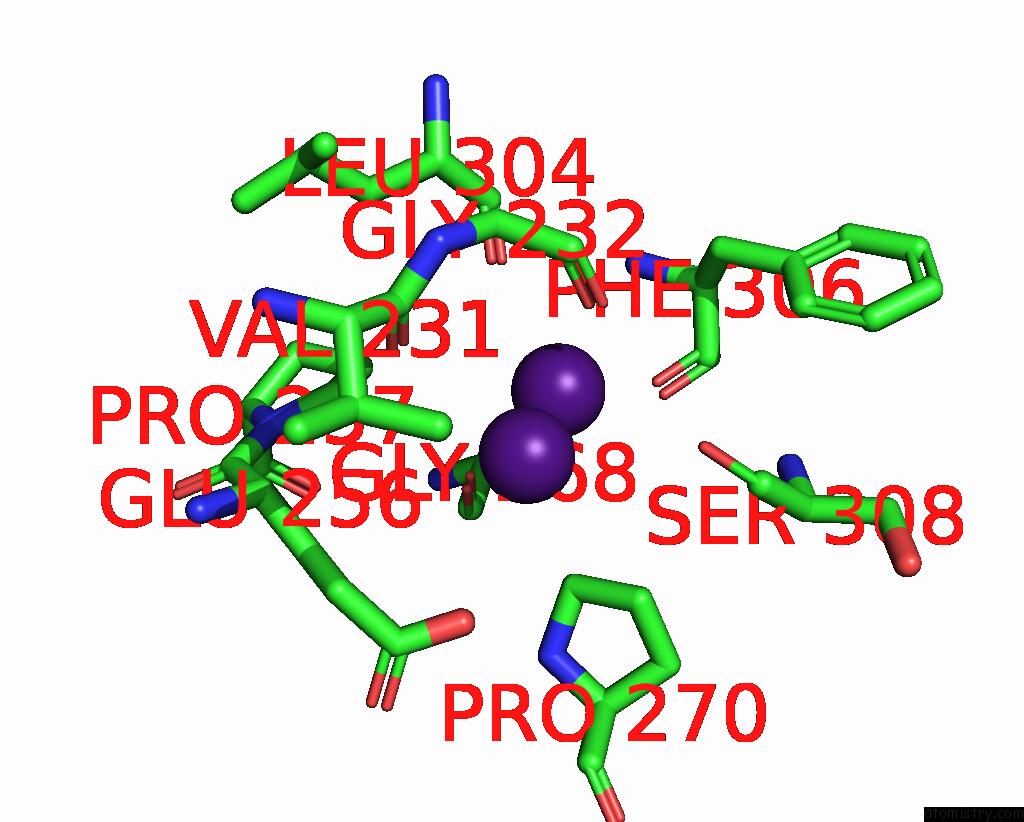
Mono view
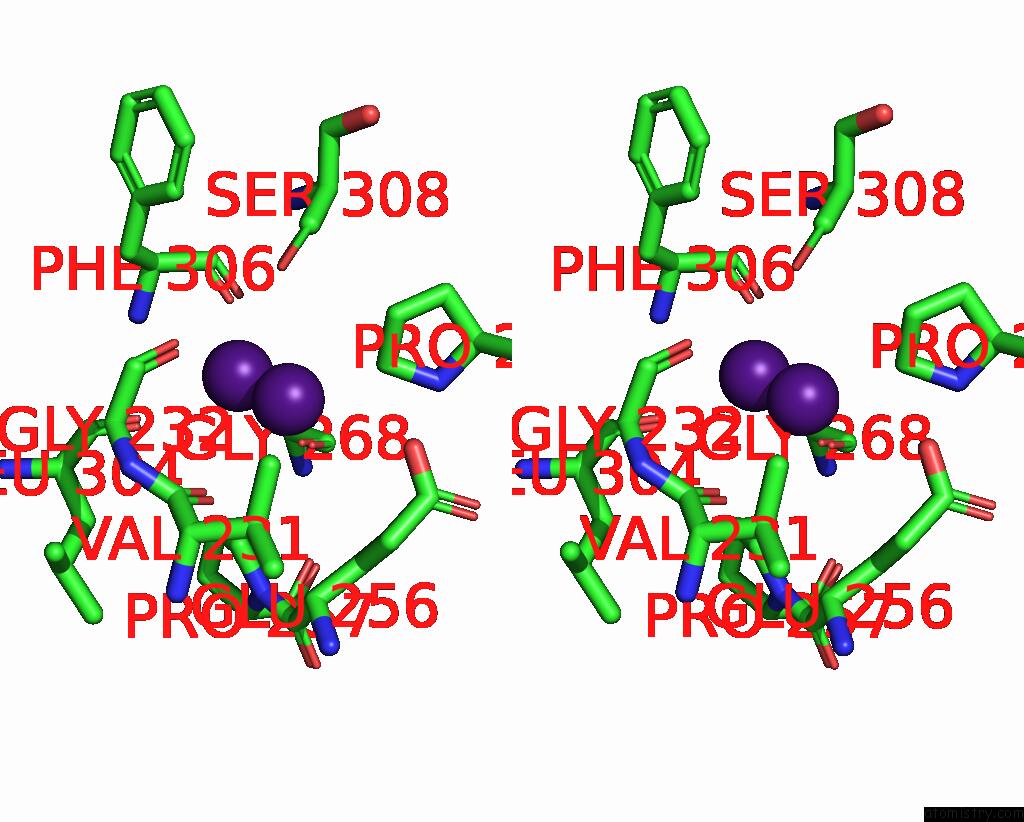
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 6 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 7 out of 8 in 7lkl
Go back to
Caesium binding site 7 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
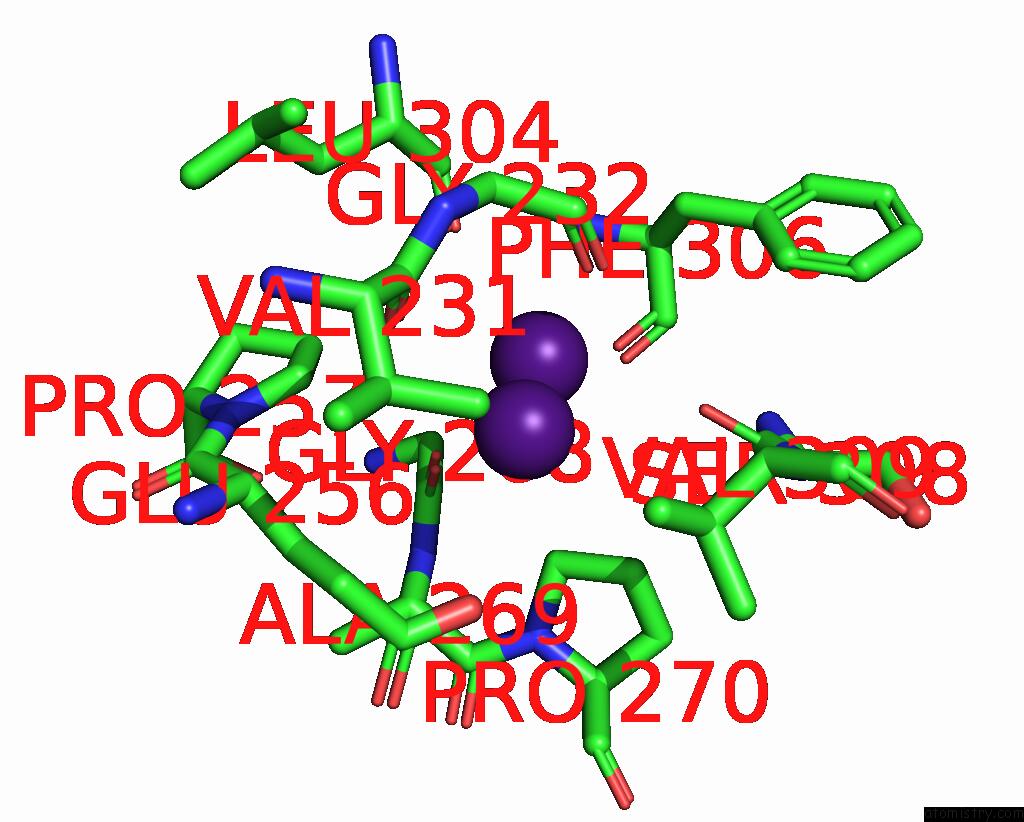
Mono view
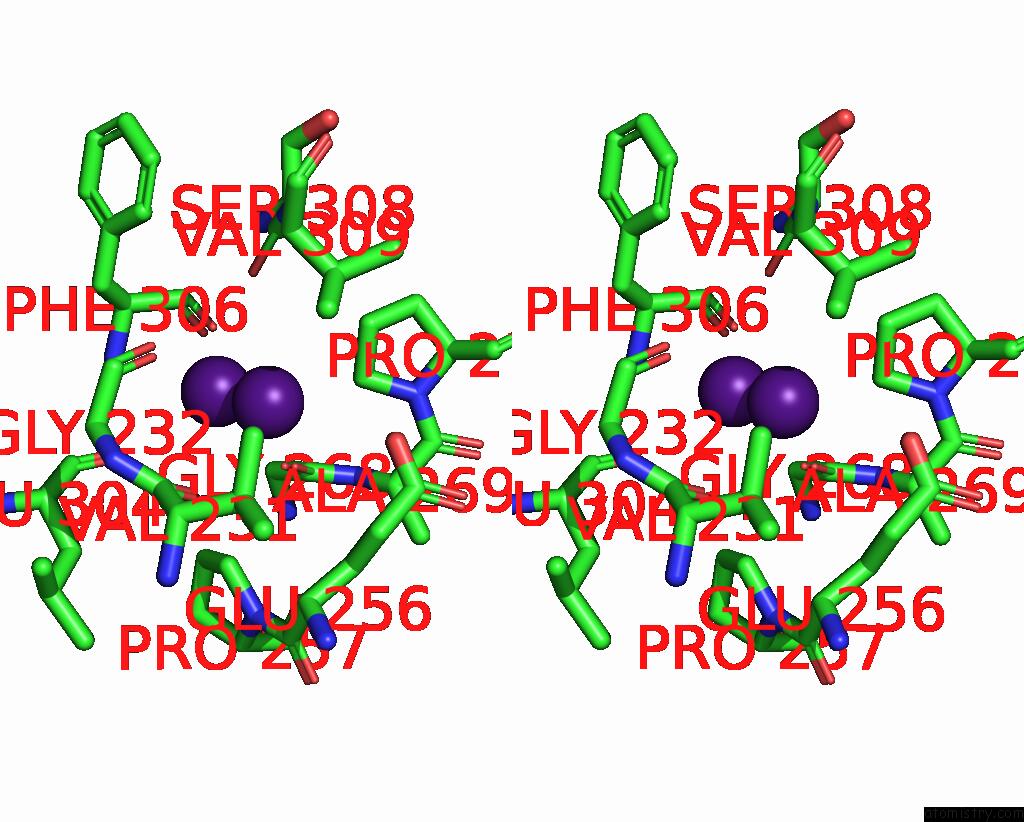
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 7 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Caesium binding site 8 out of 8 in 7lkl
Go back to
Caesium binding site 8 out
of 8 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
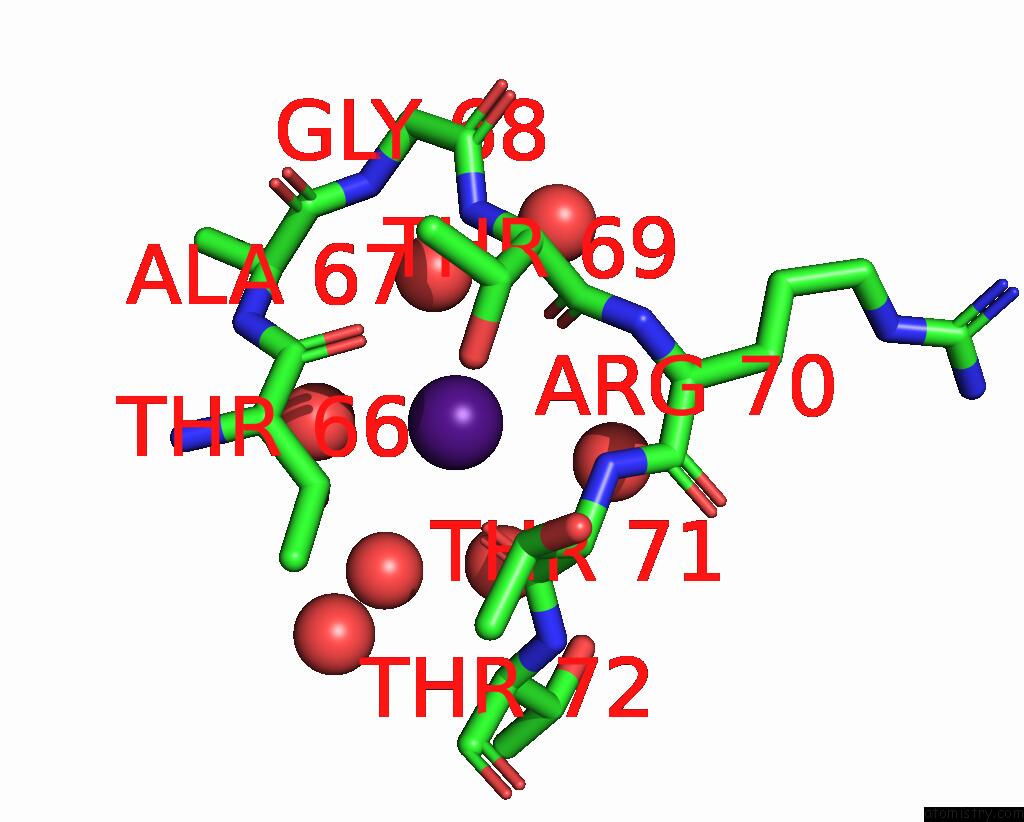
Mono view
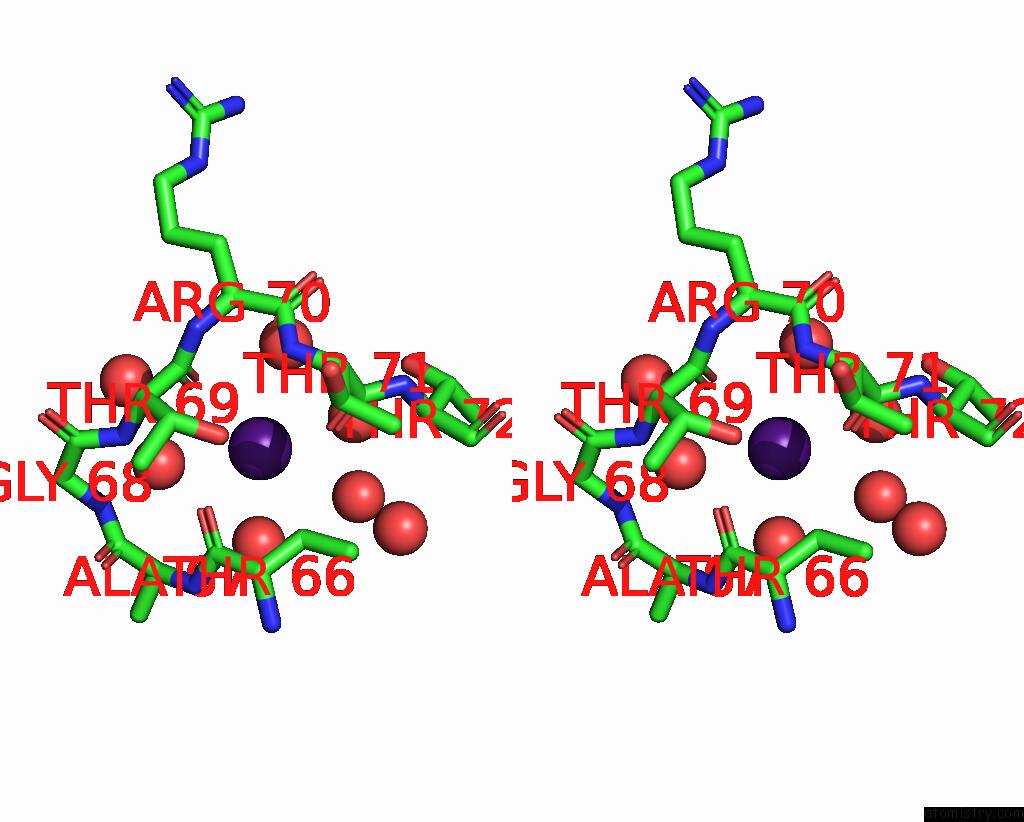
Stereo pair view

Mono view

Stereo pair view
A full contact list of Caesium with other atoms in the Cs binding
site number 8 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'- Trifluoromethoxybenzenesulfonyl)-2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Reference:
E.Hilario,
M.F.Dunn,
L.J.Mueller.
The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Inhibitor N-(4'-Trifluoromethoxybenzenesulfonyl) -2-Amino-1-Ethylphosphate (F9F) at the Enzyme Alpha-Site, Cesium Ion at the Metal Coordination Site and the Product L-Tryptophan at the Enzyme Beta-Site at 1.05 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring. To Be Published.
Page generated: Sun Jul 13 23:15:01 2025
Last articles
I in 3QD5I in 3QF1
I in 3QH9
I in 3QGN
I in 3Q7S
I in 3Q5M
I in 3Q1E
I in 3PMC
I in 3PM6
I in 3PY4